Industry Sponsored Clinical Trial Agreements (ISCTAs)
Industry Sponsored Clinical Trial Agreements (ISCTA) are the true legal binding contract for services to be provided for the industry sponsored clinical trial. They define the specific details of the Clinical Trial, including but not limited to costs, processes, and outcomes.
ISCTAs must align with the study protocol, must satisfy all regulatory requirements, institutional policies and guidelines, and the Sponsor’s guidelines. In addition, each ISCTA should define all agreed-upon costs. A clear and thorough ISCTA is vital to the success of a clinical trial, and will be an important document for both the institution and the sponsor in cases of potential liability or dispute.
SP Point of Contact:
Email: ELP-Research-Contracts@ttuhsc.edu
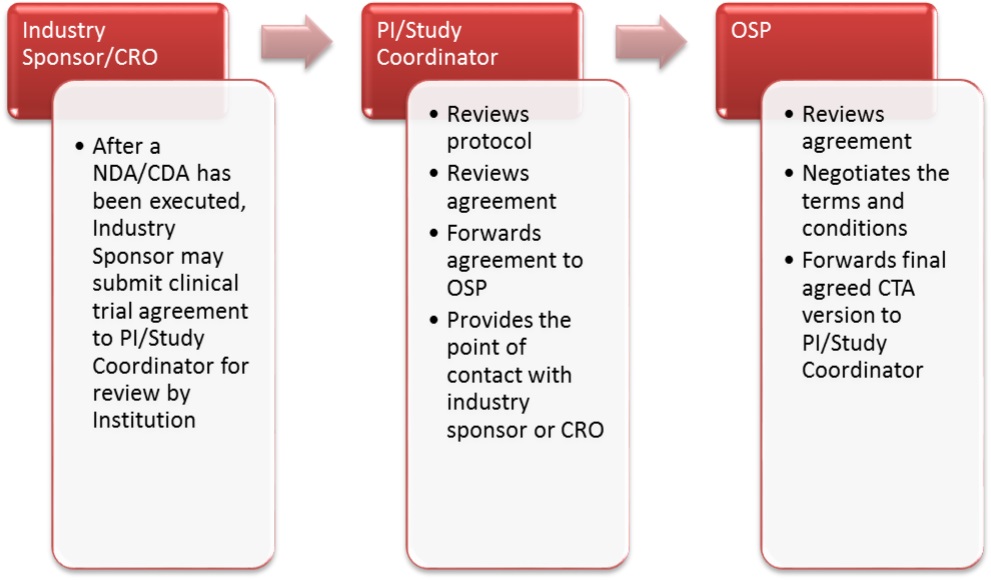
ISCTA Workflow Process