Confidentiality Disclosure Agreement (CDA) Non-Disclosure Agreement (NDA)
A Confidential Disclosure Agreement (CDA), also referred to as a Non-Disclosure Agreement (NDA), is a legal agreement between a minimum of two parties (TTUHSC EP & Industry Sponsor), which outlines information the parties wish to share with one another for research evaluation purposes.
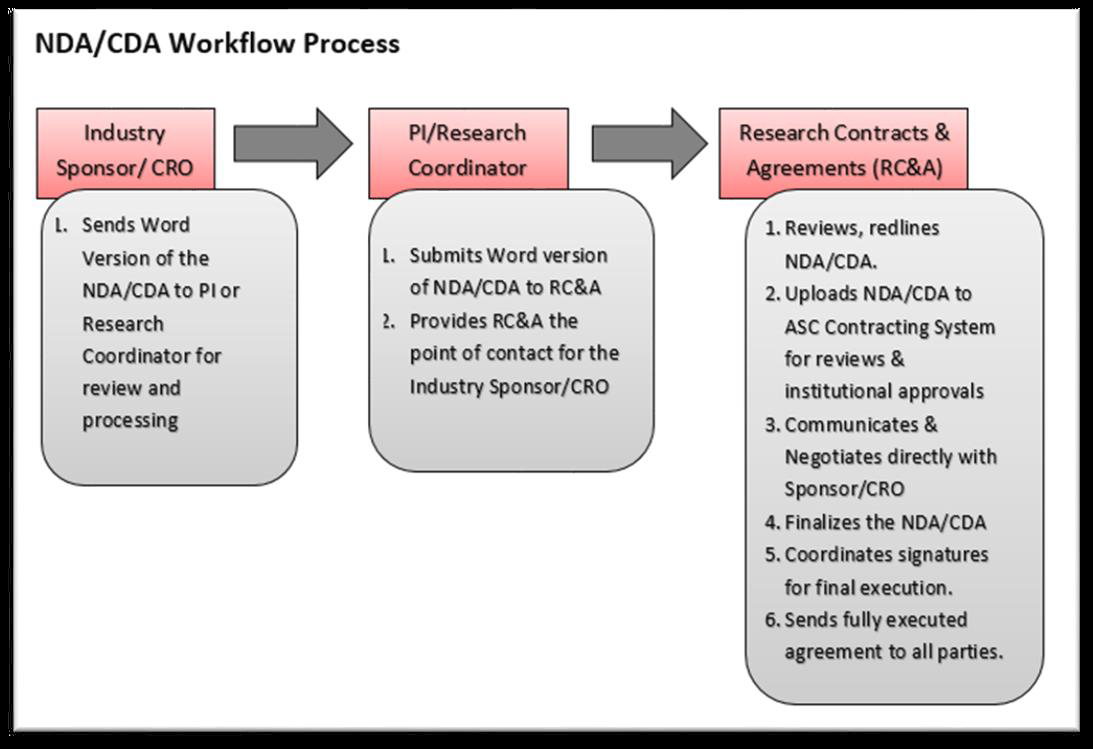
A signed, study-specific or master CDA/NDA is generally required before a sponsor will provide its proprietary information (e.g., a study protocol) to a PI. If the other party does not require a CDA/NDA, PI or Research Coordinator must obtain an email from the Industry Sponsor indicating it is not a requirement.
The terms and conditions of a CDA/NDA will be negotiated with the Industry Sponsor/ Contract Research Organization (CRO) in accordance with TTUHSC EP policies.
Research Contracts & Agreements (RC&A) is the main point of contact to assist with a Research related CDA/NDA. CDAs/NDAs are reviewed and approved by several departments at TTUHSC EP.
The content and purpose of these agreements will drive which departments will review and approve the language and terms of the agreement. Establishing the terms of the CDA is critically important since these terms most likely will define the provisions of the study-specific Industry Clinical Trial Agreement.
Research Contracts & Agreements Point of Contact:
ELP-Research-Contracts@ttuhsc.edu